Maximizing respiratory trial outcomes: From primary to exploratory measures
Quantitative evaluation of medical images plays an increasingly important role in research and clinical trials for drug development. Imaging-based outcomes reveal deeper insights into pulmonary diseases and allow for an earlier identification of abnormalities, with much better accuracy and precision, in comparison to traditional pulmonary function test methods.
Our advanced AI-powered chest CT analysis provide fast and robust quantitative assessment of all major lung pathologies.
In multi-site and longitudinal trials, where managing imaging variability is a major challenge, AI can help standardize analysis, ensuring consistent and reproducible assessments across different locations and time points.
By enhancing primary outcomes with objective, quantitative biomarkers, strengthening secondary measures with detailed structural and functional insights, and facilitating novel biomarker discovery, pharmaceutical companies can gain a strategic advantage in running more efficient, data-driven, and regulatory-aligned respiratory trials.
Robust CT outcome measures with Artificial Intelligence
Our AI-powered analysis of chest CT scans help pharmaceutical companies streamline clinical trials across different phases of drug development in a wide range of rare and common lung diseases, including COPD, bronchiectasis disease, asthma, ILD, Cystic Fibrosis and other.
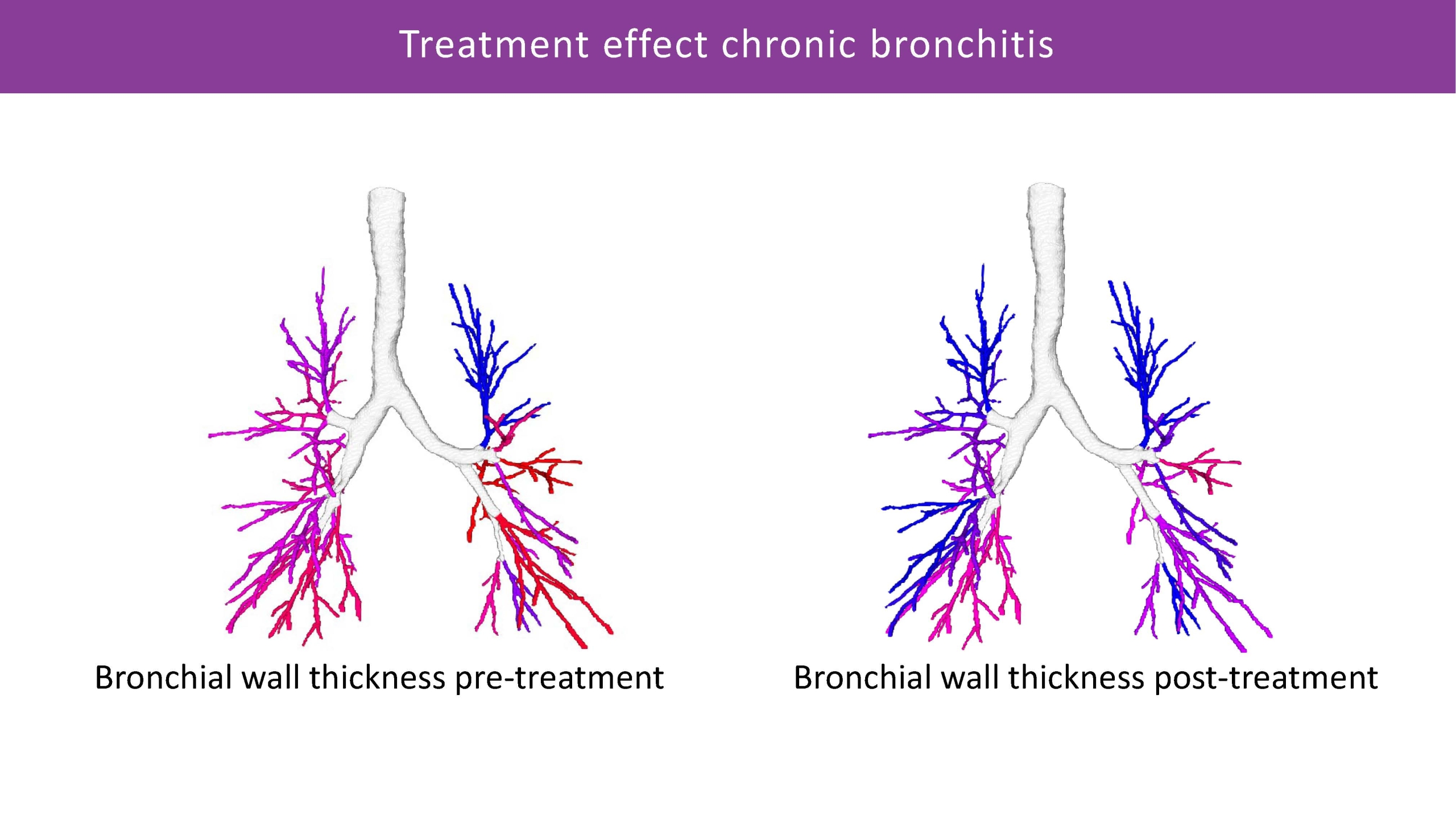
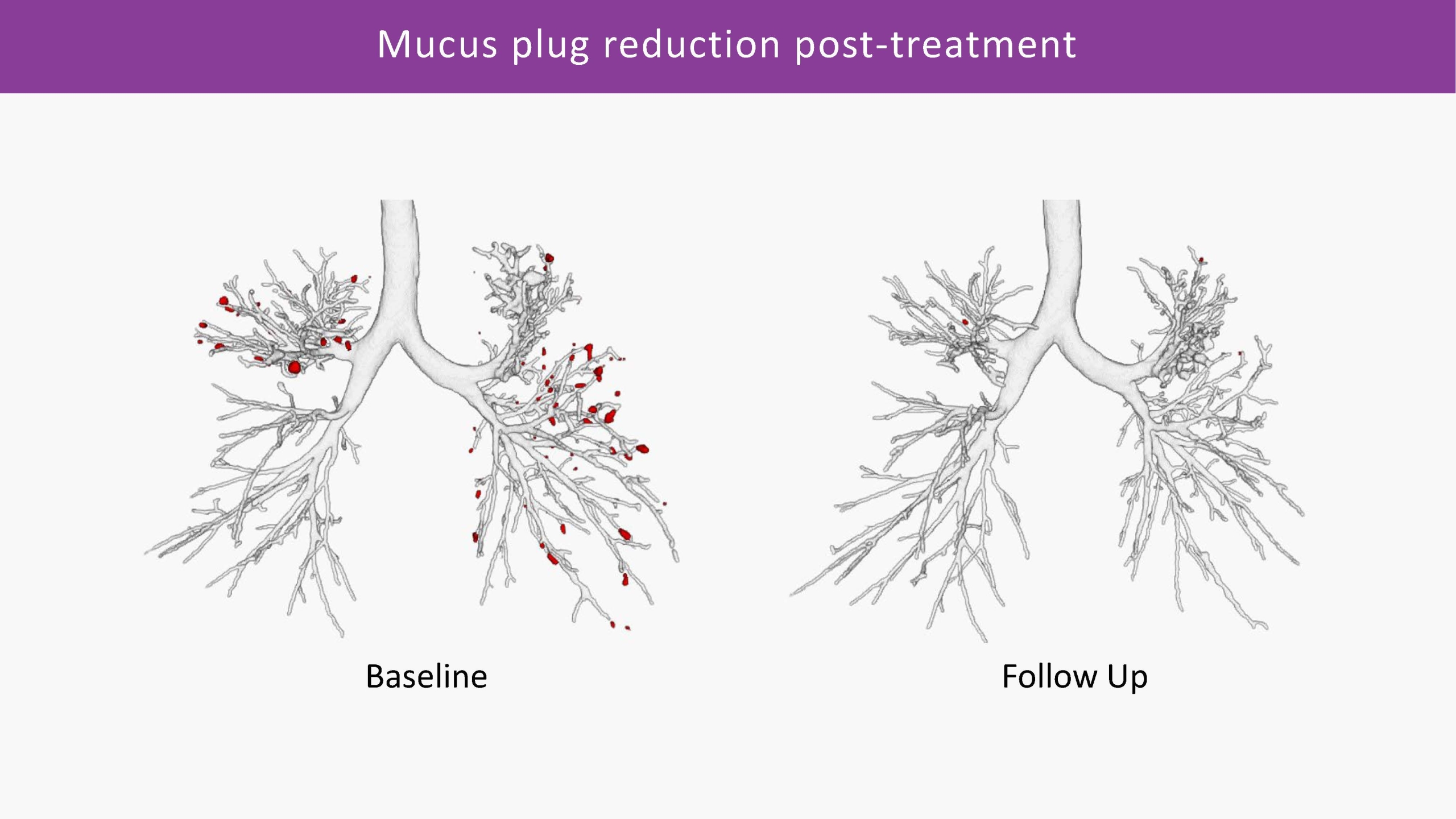
Combining advanced segmentation and quantification capabilities for analysing lung structures, airway and vessel dimensions and parenchymal density, we can provide detailed local assessment of emphysema, air trapping, bronchiectasis, mucus impaction, and other conditions.
The objective data generated by Artificial Intelligence, can help significantly improve trial efficiency and strengthen regulatory approval process. In Phase I and II trials, imaging can help detect early treatment signals and refine patient selection. In Phase III and IV, it may offer more precise efficacy assessments and support long-term disease monitoring.
Learn more on our LungQ technology platform and the measurement portfolio.
*Thirona’s LungQ® technology platform offers a portfolio of measurements and analytical capabilities intended either for clinical use or clinical research. Availability and intended use of the analysis may vary by geography. Please contact us for further information regarding the latest regulatory status in your specific market and its applicability to your use case.
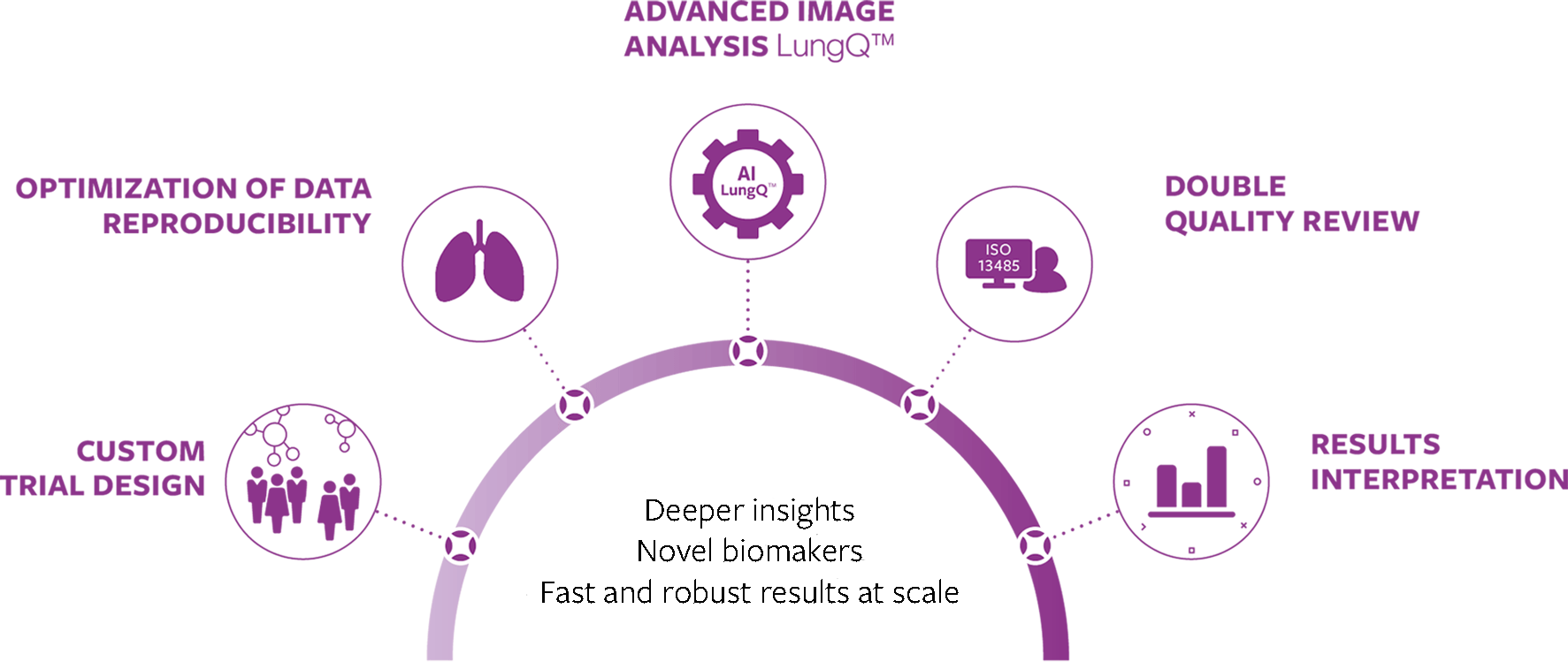
Supporting the full process towards optimal end results
Clinical validation and scientific evidence
Thirona’s algorithms are being continuously validated across a wide range of lung diseases and patient populations, leveraging our strong network of partnerships with academia, academic hospitals, research organizations, and patient registries.
This ongoing evaluation includes comparisons with traditional assessment methods, multi-center studies, and real-world performance evaluations, ensuring high standards of accuracy, reproducibility, and clinical applicability in diverse settings.
For a detailed overview of our validation studies and published research, visit our Publications Page.
Partnering for integrated imaging service in respiratory clinical trials
In collaboration with established CROs and trial imaging management platforms, we can offer clinical trial sponsors the option to access Thirona’s LungQ® analyses through coordinated imaging workflows with their preferred providers, when specified by the protocol.
Sponsors can retain the operational efficiency of their existing data pipelines while requesting selected LungQ® measurements for advanced quantification of bronchial, parenchymal, and vascular structures. This integral service model supports fast, reliable delivery of reproducible quantitative outcome measures and helps minimize operational risk and time-to-market.
Partners we work with: