What if one often underestimated step in image acquisition could determine the success of your clinical trial outcomes? In this blog, we explore how lung volume control can critically influence the reproducibility and sensitivity of imaging results – and, ultimately, the overall success of imaging-based respiratory trials.
Poor Input, Poor Output: What Isn’t Captured in the Scan Can’t Be Quantified
In longitudinal pulmonary studies, where CT is used to assess disease progression or therapeutic response, even small changes in airway dimensions or parenchymal density can carry significant clinical meaning. For these changes to be captured reliably, scans must be acquired at comparable inspiratory and expiratory lung volumes.
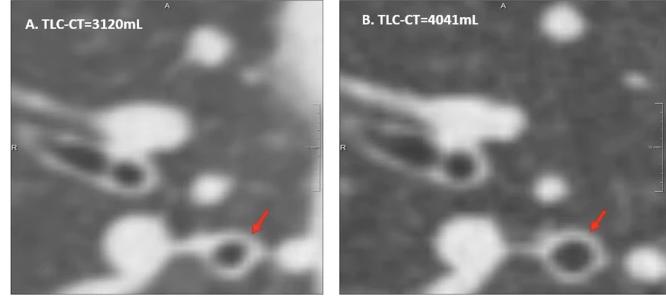
Comparison of two inspiratory CT slices from the same patient in one session, acquired at different lung volumes.
Image A: Scan at 3120 mL; Image B: Scan at 4041 mL.
Both show the RB1 region, illustrating substantial differences in bronchial diameters (red arrow) due solely to differences in inspiratory volume.
[Source: Chen et al., European Radiology, 2024]
Insufficient inspiration reduces airway dimensions, leading to underestimation of bronchial dilatation and overestimation of wall thickness. Likewise, suboptimal expiration can mask gas trapping. This volume-related variability doesn’t just complicate image interpretation – it can bias AI-generated metrics, misrepresent patient trajectories, and compromise study endpoints.
In short, AI algorithms perform optimally when lung volumes are standardized and optimized.
The Challenge That Can Be Addressed
Over the past two decades, the importance of lung volume control – both at total lung capacity (TLC) and residual volume (RV) – has been well established. A pioneering protocol at Erasmus MC-Sophia Children’s Hospital in Rotterdam introduced spirometer-guided chest CT and MRI scanning as early as 2007. Designed to ensure consistent lung volume in children with cystic fibrosis, the approach combined pre-scan training and real-time volume control during image acquisition.
The results, published by Salamon et al. (Pediatric Pulmonology, 2017), demonstrated that even children as young as five could consistently achieve high-quality, reproducible scans. This method remains standard practice for all chest CTs at the hospital today.
Building on this foundation, a recent study by Chen et al., published in 2024 in European Radiology, examined how inspiratory volume levels affect airway and vascular measurements in adult COPD patients. Notably, the study used Thirona’s LungQ Bronchial-Artery (BA) software, highlighting the critical value of consistent lung volume for quantitative imaging analysis in adult populations as well.Side-by-side comparison of CT scans at inspiration and expiration, including corresponding AI-based analysis using LungQ-BA. The images demonstrate how differences in lung volume impact airway detection and measurements, influencing the number of visible bronchi and the resulting analysis outcomes.
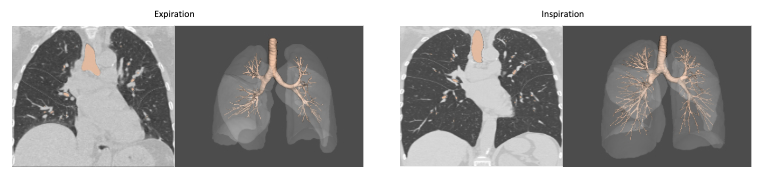
Even the Most Powerful Algorithm Depends on Proper Image Acquisition
Thirona’s LungQ algorithms, enabling a wide range of bronchial, vascular, and parenchymal analysis, are built to handle real-world imaging variability, and their consistently high performance is backed by a multitude of validation studies. However, LungQ delivers its most accurate and clinically meaningful results when lung volume is properly optimized (for both TLC and RV).
When paired with well-executed volume control, a high-quality algorithm can extract the maximum diagnostic and quantitative value from every scan – crucial for generating robust imaging outcomes in clinical trials – and provide:
- Accurate quantitative assessment of airway dimensions and wall thickness;
- Sensitive detection of gas trapping and ventilation heterogeneity;
- Reliable tracking of structural abnormalities, over a wide range of bronchial generations.
Reducing lung volume variability is key to optimizing the sensitivity and reproducibility of CT-based outcomes, especially when assessing therapeutic effects on bronchial dimensions in lung disease.For pharmaceutical sponsors, CROs, and academic researchers, reducing lung volume variability is key to ensuring sensitivity and reproducibility in CT-based outcomes – especially when studying therapeutic effects on bronchial dimensions in COPD, bronchiectasis disease, severe asthma, or interstitial lung disease.
Raising the Bar in Respiratory Clinical Trials
As AI becomes the cornerstone of quantitative imaging in respiratory trials, volume standardization is no longer optional – it’s essential. At Thirona, we work closely with trial sponsors and imaging partners to support best practices in scan acquisition, because when data quality improves, so does the value of every AI-powered insight.
With standardized lung volume control, imaging trials can rely on:
- Accurate and sensitive comparison between baseline and follow-up scans;
- Greater reproducibility of AI-generated measurements;
- Improved detection of subtle treatment effects;
- Higher statistical power in evaluating interventions.
And implementation is practical, most importantly including pre-scan training to rehearse breath-hold maneuvers and on-site coaching from lung function technicians during the scan, and if feasible spirometer-guided acquisition to ensure scans are taken at the correct lung volume. This approach significantly improves image quality and consistency – even in young children and dyspneic populations – without disrupting workflow.
Ready to Raise the Bar?
Contact us to explore how Thirona can support your trial with standardized, AI-enhanced lung imaging and help you unlock the full potential of imaging outcome measures.
