Statistically powerful clinical trials with medical imaging AI
Quantitative evaluation of medical images plays an increasingly important role in research and clinical trials for drug development. Imaging-based outcomes reveal deeper insights into pulmonary diseases and allow for an earlier identification of abnormalities, with much better accuracy and precision, in comparison to traditional pulmonary function test methods. Our advanced solutions for AI-powered chest CT analysis provide a fast and detailed quantitative assessment of all major lung and respiratory pathologies.
Wide range of extensively validated and novel measurements
Our modular platform contains generalizable deep learning models developed for anatomical and disease-related quantification tasks in a multitude of diseases.
Advanced anatomical segmentation algorithms of the lungs, lobes, pulmonary (sub)segments, interlobar fissures, airways and anatomical branches, pulmonary arteries and pulmonary veins allow for very precise and detailed exploration of local lung pathologies for both common and rare pulmonary diseases such as COPD, Asthma, COVID-19, Cystic Fibrosis, Interstitial Lung Disease, Pulmonary Arterial Hypertension and more.
Thirona's artificial intelligence-based quantification capabilities for chest CT analysis, include:
- Anatomical measures such as: volumes of each anatomical structure, parenchymal density evaluation, bronchial and vascular dimensions, bronchus-artery analysis (BA), fissure completeness, artery-vein phenotyping (AVX), etc.
- Disease-related severity and distribution assessment for: emphysema, air trapping, bronchial wall thickening, bronchial and vascular dropout, covid-19 infection, bronchiectasis, fibrosis, mucous impaction, atelectasis, CT-approximated perfusion (PXT) and ventilation defects, pulmonary vascular enlargement, etc.
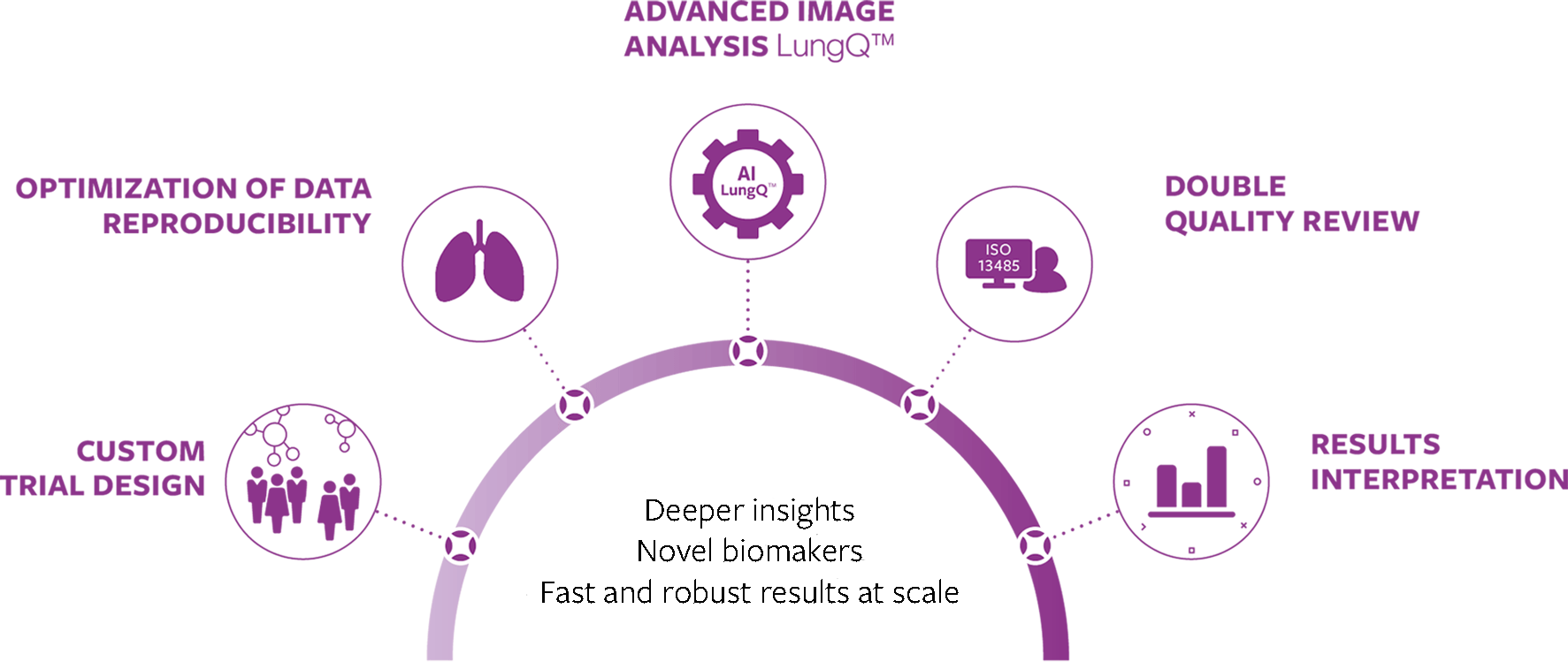
Supporting the full process towards optimal end results
Certified solution
LungQ 3.0.0 clinical software package
Approved for clinical use in Europe and USA
The Thirona’s LungQ v3.0.0 software produces CT values for pulmonary tissue, providing quantitative support for diagnosis and follow-up examinations. LungQ can be used to support the physician in the diagnosis, evaluation and documentation of pulmonary tissue images (e.g. abnormalities) from CT thoracic datasets. The analysis provided includes segmentation and isolation of sub-compartments, volumetric analysis, density evaluations, fissure evaluation and reporting tools.
LungQ v3.0.0 clinical software is FDA 510(k) cleared and a certified as a class IIb medical device CE marked (CE 0344) under the European Medical Devices Regulation (EU-MDR 217/745). The CE certified version provides additional analysis of tissue destruction and airway and vessels evaluation.
Partnering for the shortest path to the highest quality imaging endpoints
Clinical trials often face unique challenges, from patient phenotyping and defining the best fit-for-purpose measurement, up to delivering robust evidence – it all needs to be done with no compromise to make sure trials are effective.
Our integral approach ensures fast and reliable delivery of precise imaging endpoints, while minimizing costs and time-to-market.
Full scope imaging service

In partnership with Voiant, a clinical trial imaging solution provider, we offer a fully integrated service allowing to run clinical trials with imaging effectively, while minimizing risk of trial failure and saving on operational expenses.
With the unique benefit of our combined expertise in lung image analysis and clinical research, proven technology platform and well-guarded quality assurance process along the whole trial, we can guarantee robust results at any scale, with consistent output quality. One integral approach, one platform, one contractor for seamless operations and reliable execution.


