Advancing Clinical Trials for Pharmaceutical Drug Development
Imaging-based insights
across trial pathways
AI-powered quantitative chest CT analysis is increasingly vital in respiratory clinical trials, providing non-invasive, precise, and objective assessment of anatomical abnormalities and disease severity to complement traditional endpoints.
Whether supporting primary, secondary, or exploratory endpoints, LungQ® metrics enable in-depth exploration of treatment effects and mechanism of action.
With accurate, sensitive, and reproducible measurements, our advanced LungQ® ensures consistent, evidence-based insights across the entire trial pathway.
pre- clinical trial
Patient phenotyping
and selection
phase 1
Early evidence on treatment
response patterns
phase 2-3
Quantitative data on treatment
efficacy and disease mechanisms
phase 4
Longitudinal evidence of sustained treatment outcomes
Why sponsors choose our analysis
Consistent output quality
handling large data sets variability across trial sites
Strong clinical correlation
in comparison to conventional methods and clinical parameters
Extensive validation across diseases
in diverse patient populations from adult to pediatric age groups
Advanced QCT analysis with artificial intelligence
Combining high-fidelity segmentation and quantification capabilities, LungQ analysis provides a comprehensive structural profile characterization of the lung, including airways, blood vessels, and parenchyma, delivering granular insights at the regional level. This enables precise, sensitive quantification of emphysema, trapped air, bronchiectasis, mucus impaction, and related patterns, adding objective evidence alongside clinical and functional readouts.
Our areas of expertise include COPD, bronchiectasis disease, severe asthma, cystic fibrosis (CF), interstitial lung disease (ILD), and other rare and common respiratory conditions, in both adult and paediatric populations.
Examples of QCT analyses with LungQ®:
Bronchial thickening
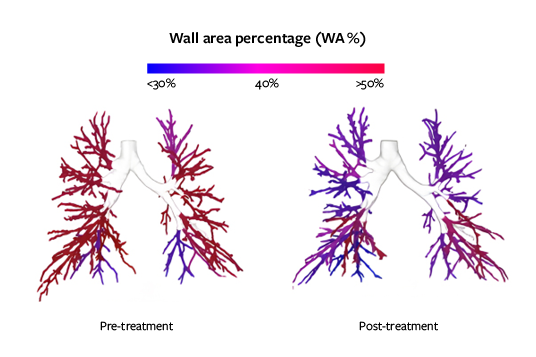
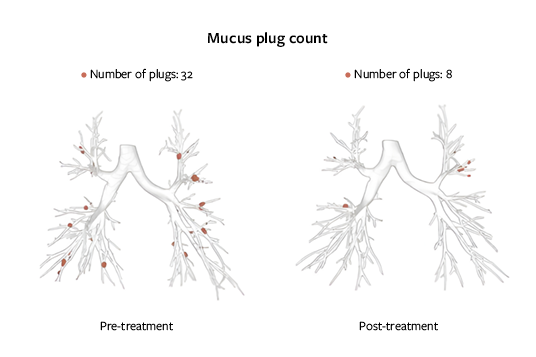
MUCUS IMPACTION
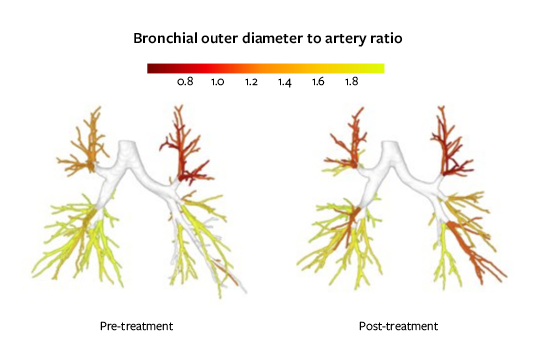
Airway narrowing/widening
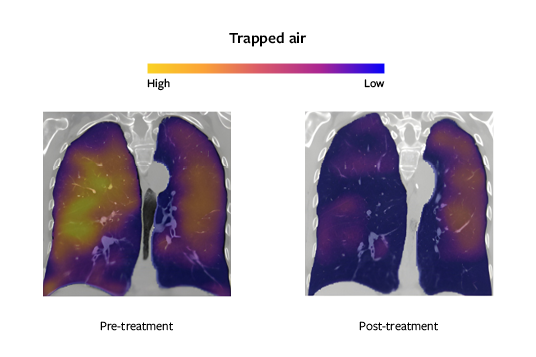
Ventilation estimation of small airways
Note: Thirona’s LungQ® technology platform offers a portfolio of measurements and analytical capabilities intended for either clinical use or clinical research . Availability and intended use of the analysis may vary by geography. Please contact us for further information regarding the latest regulatory status in your specific market and its suitability for your intended use.
Unlocking full imaging potential
Beyond automated analysis, we offer enhanced expertise to help sponsors extract the full value from their imaging studies. From guiding protocol choices and standardizing image acquisition to extending insights after AI analysis, our tailored support ensures optimized data quality, impactful results, and stronger trial outcomes.
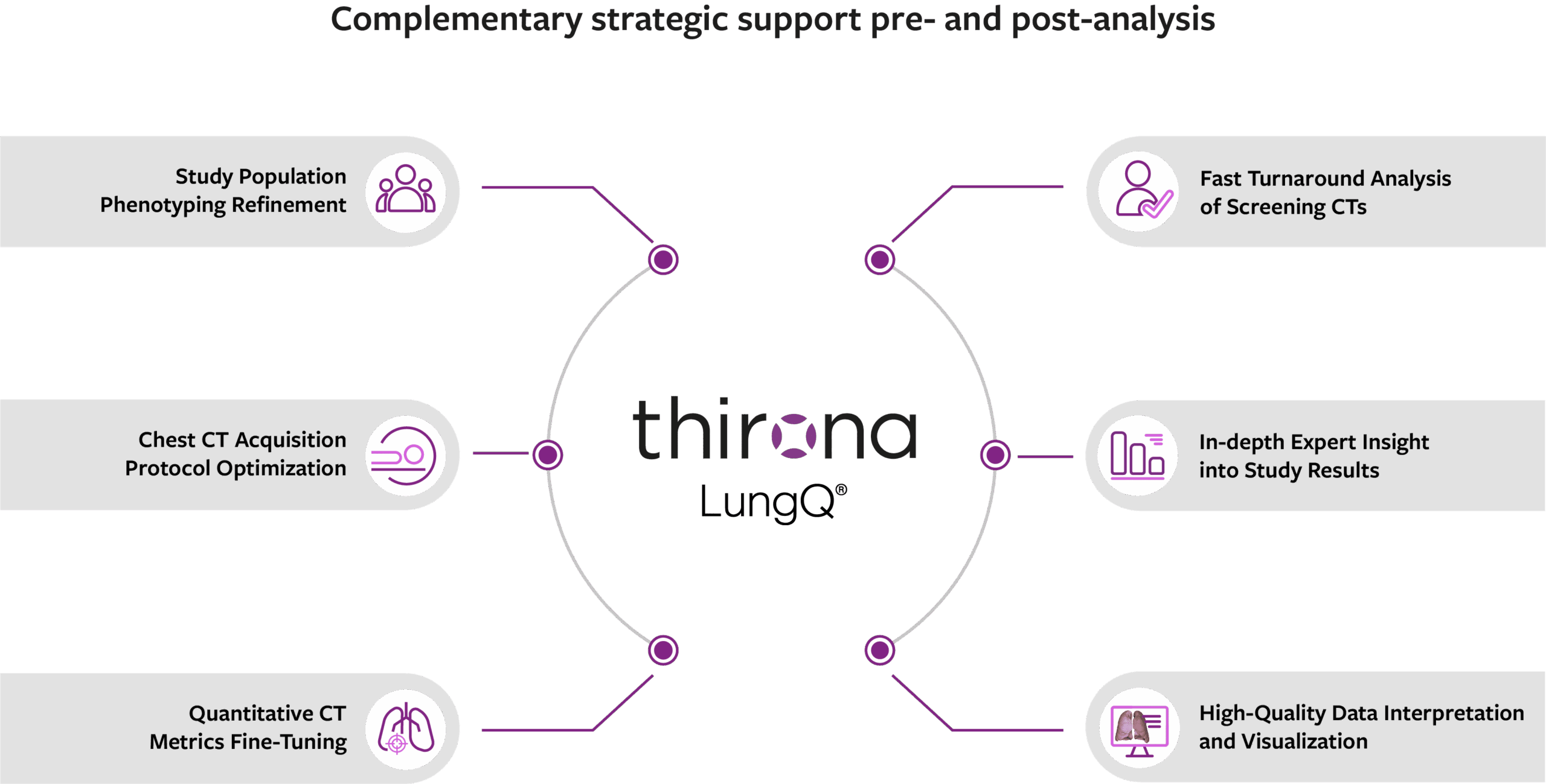
Use cases accelerating precision medicine
Precise quantification of pulmonary structural abnormalities
LungQ analyses have been demonstrated to deliver sensitive and accurate quantification of bronchial, parenchymal, and vascular abnormalities at both global and local levels across major respiratory diseases. The precision even exceeds established visual and expert-driven clinical methods.
Beyond traditional benchmarks, LungQ® metrics provide deeper quantitative insights into inter-and intra-patient heterogeneity, facilitating a high degree of personalized medicine whether for precise patient phenotyping or the planning of localized treatments.
Furthermore, LungQ excels at the earlier detection of subtle structural and functional changes during disease stages. This sensitive early-warning capability enables clinicians to take actions sooner, potentially altering the disease trajectory before irreversible damage occurs.

Validation studies
European Respiratory Journal
| January 2026
Lobar differences on chest CTs of bronchiectasis patients from the EMBARC registry
American Thoracic Society Conference
| May 2025
Assessment of 10-year Progression in COPDGene Using Automated Measurements of Bronchus-Artery Ratios and Mucus Plugging
Journal of Cystic Fibrosis
| January 2026
Progression of structural lung disease and lung function in adolescents with cystic fibrosis
European Respiratory Journal
| January 2026
Automated measures of bronchial outer diameter tapering across different COPDGene GOLD classes
European Respiratory Journal
| January 2026
AI-measured bronchial dimensions and emphysema: A duo for predicting airflow limitation in COPD
European Respiratory Journal
| January 2026
COPD progressors vs. non-progressors: 10-year change in Bronchus-Artery ratios & mucus plugs
Thorax
| September 2023
Automatic Analysis of Bronchus-Artery Dimensions to Diagnose and Monitor Airways Disease in Cystic Fibrosis
European Respiratory Journal (ERS Congress)
| October 2024
Automatic analysis of bronchus-artery ratios and mucus plugs of 640 chest CTs of EMBARC bronchiectasis patients
ERJ Open Research
| August 2025
Automated computed tomographic analysis of bronchial thickness and mucus plugs in bronchiectasis with asthma
Sensitive assessment of pharmaceutical treatment response
LungQ® metrics provide sensitive imaging endpoints for anatomical abnormalities by delivering standardized, automated measurements, enabling a precise and objective evaluation of treatment efficacy and longitudinal follow up across diverse pharmaceutical interventions such as mucolytic therapies, anti-inflammatory therapies and CFTR modulators.
Whether serving as primary or secondary outcome measure, those metrics provide deep and localized insights for clinical trials, real-life studies and observational studies.
Furthermore, By detecting subtle structural response, LungQ® analyses can help researchers to optimize their trial design and improve productivity.

Validation studies
The Lancet Respiratory Medicine
| October 2025
Effect of elexacaftor–tezacaftor–ivacaftor on bronchial dilatations in adolescents with cystic fibrosis: a multicentre prospective observational study
European Respiratory Journal
| January 2026
Structural lung abnormalities in children and adults with cystic fibrosis after ETI using AI automated algorithm
European Respiratory Journal
| January 2026
AI-based mucus plug analysis to evaluate the effect of inhaled hypertonic saline in preschool children with CF: a randomized controlled trial
European Respiratory Journal
| January 2026
The effect of Elexacaftor/Tezacaftor/Ivacaftor (ETI) on bronchial tapering as marker of bronchial dilatation in people with CF aged 12 above (RECOVER study)
Journal of Cystic Fibrosis
| May 2023
Automatic Bronchus and Artery Analysis on Chest Computed Tomography to Evaluate the Effect of Inhaled Hypertonic Saline in Children Aged 3-6 Years With Cystic Fibrosis in a Randomized Clinical Trial
Pediatric Pulmonology
| January 2025
Introduction of Ivacaftor/Lumacaftor in Children With Cystic Fibrosis Homozygous for F508del in the Netherlands: A Nationwide Real-Life Study
Research Square
| October 2025
The impact of dornase alfa on imaging features of bronchiectasis
Respiratory trial population refinement through advanced phenotyping insights
Recognizing the substantial patient heterogeneity in respiratory disease populations, LungQ® analyses enables precise patient phenotyping by identifying specific lung phenotypes or regional disease distribution that conventional methods often overlook, allowing researchers and clinicians to align patient profiles with stringent inclusion and exclusion criteria and ensuring that clinical trial cohorts are enriched with the structural abnormalities most relevant to the therapeutic mechanism.
This targeted approach accelerates the delivery of personalized medicine by reducing “noise” in trial data and focusing on populations where the treatment is most likely to demonstrate a significant clinical impact.

Validation studies
European Respiratory Journal
| January 2026
Lobar differences on chest CTs of bronchiectasis patients from the EMBARC registry
American Thoracic Society Conference
| May 2025
Assessment of 10-year Progression in COPDGene Using Automated Measurements of Bronchus-Artery Ratios and Mucus Plugging
ERJ Open Research
| December 2024
Automated method of bronchus and artery dimension measurement in an adult bronchiectasis population
Partnering for the shortest path to effective trials
In collaboration with established CROs and trial imaging management platforms, we can offer clinical trial sponsors the option to access Thirona’s LungQ® analyses through coordinated imaging workflows with their preferred providers.
Sponsors can retain the operational efficiency of their existing data pipelines while requesting selected LungQ® measurements for advanced quantification of bronchial, parenchymal, and vascular structures. This integral service model supports fast, reliable delivery of reproducible quantitative outcome measures and helps minimize operational risk and time-to-market.
Partners we work with:




