Rudolfs Latisenko, Senior Deep Learning Engineer at Thirona, explains Thirona’s capabilities in artificial intelligence-based airway analysis of chest CT images: How it works and what insights can be generated with a consistent accuracy and reliability.
Why are airways important to objectively assess lung disease?
Airways play a central role in the functioning of the respiratory system. The whole mechanism of breathing happens through them, which makes it a crucial structure to assess when approaching lung disease. Different diseases have different effects on the airways, so measuring them allows us to understand their contribution to the underlying disease process.
Why is airway assessment from CT scan a challenge?
Airways can be challenging to assess accurately from a CT scan.
They can show different structural changes in relation to the underlying lung disease - narrower and less visible on scans in obstructive diseases like COPD (Chronic Obstructive Pulmonary Disease), Asthma, or thickened, widened, or obstructed by mucus in other diseases involving a great deal of bronchiectasis, like CF (Cystic Fibrosis), or IPF (Idiopathic Pulmonary Fibrosis).
In addition to the mentioned abnormalities caused by the disease, airways vary a lot in size within a scan due to their tree-like structure, getting smaller with each branching. There are also multiple anatomical variations in the airway tree structure that can make them quite unique between patients.
Furthermore, the technical aspects of CT acquisition like the patient's level of inspiration, characteristics of reconstruction kernel, and radiation dose can all affect the visibility of airways on the scan.
And finally, there are a lot of airways visible on a CT scan. They are challenging to spot and assess with the naked eye. For instance, in adult patients at a proper inspiration level there can easily be at least 800 individual branches identified, including both the larger central airways, as well as the smallest peripheral airways. This means that addressing the whole airway tree with manual methods is difficult and extremely time consuming.
What new possibilities does AI bring?
Accurate assessment of airways on a CT scan requires thorough expertise and a robust approach to identify subtle abnormalities and distinguish between time points, patients and diseases. While CT scans alone offer insights based on three-dimensional imaging, they don't provide functional and objective measurements, which is needed to assess the full extent of disease progression and airway impaction in an accurate way.
Because of recent advancements in artificial intelligence, Thirona is able to develop algorithms that tackle all the aforementioned challenges. We do this by carefully selecting the datasets used for training and validating the algorithms, making sure they represent the wide range of variation in all the different aspects we would expect during deployment.
The 20+ years of experience that Thirona’s team has in chest CT analysis has allowed us to develop tools and procedures for acquiring very high-quality annotations - the backbone of any powerful AI algorithm.
Next to high accuracy and reliability of the technology, ongoing external validation studies play a vital role in assessing the ability of our algorithms to perform consistently on diverse patient populations. We pride ourselves on the scientific research and publications that speak toward the robustness and clinical applicability of our developments.
We no longer live in a time where we need to depend on the subjective assessment of airway wall thickness or airway widening, as they can now be objectively measured and quantified. Until recently this could only be done by technicians, which would take days if not weeks to collect airway measurements on just a handful of patients.
The age of AI has brought forth developments that allow us to analyse airways from a CT scan completely automatically and with high accuracy, in the time it takes you to get a cup of coffee. In the hands of clinicians, this precise and detailed structural assessment can be used for gathering insights on disease progression allowing further treatment planning . Pharmaceutical and medical companies can now test the efficacy of new drugs more accurately and use insights that were previously not available, to develop new treatments and more advanced interventions.
Diving in – how we perform bronchial assessment with LungQ
When approaching bronchial analysis, we go through three pillars of assessment: Identification, Localization and Quantification.
Identification – airways, mucus and arteries
The process of bronchial analysis starts with identifying airways up on a CT up to a diameter of 2 mm (which we define as bronchi, as they will largely contain cartilage). Proper identification of the bronchi is crucial, forming the basis for all following steps. And with the examples below (Figure 1) you can judge the value of this even qualitatively – bronchial trees can look very different in different disease populations. Good segmentation already allows us to see some important characteristics and extract high-level metrics like branch count and tree length.
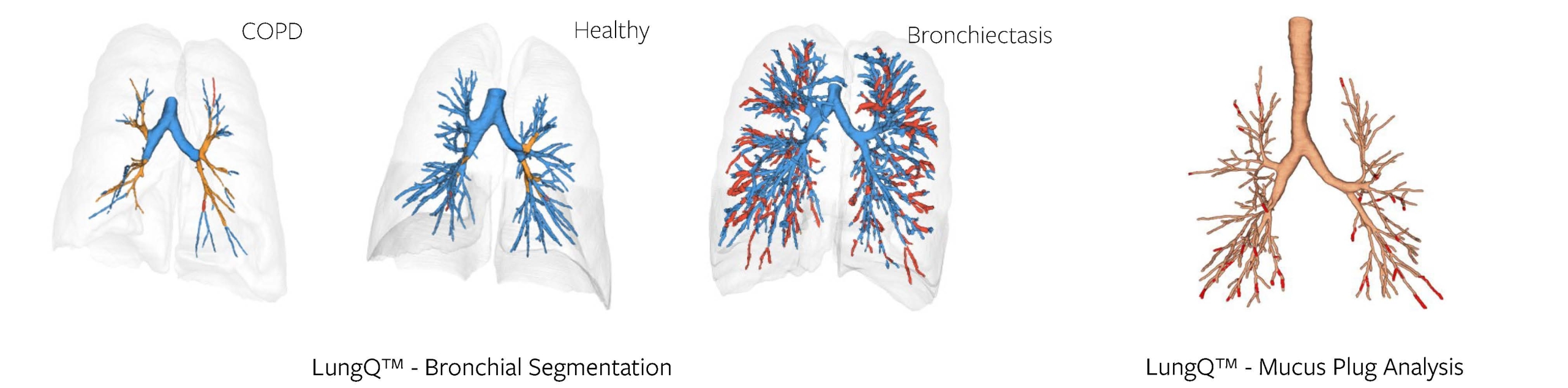
Figure 1, 2
In addition to this, we are also able to identify bronchi that are obstructed by mucus, i.e., mucus plugs, as seen in the example above (Figure 2). This is a much more difficult task, if done manually, as mucus plugs can be very difficult to detect. With the help of AI algorithms, we can identify and count them, as well as provide more detailed markers that utilize the methods of localization and quantification of the bronchial tree - described further.
Finally, for every identified bronchial branch, our algorithms can detect its adjacent artery. The arteries run parallel to the bronchi within the lungs, and the ratios between the sizes of these neighboring structures are well-known indicators used in radiological reads.
Localization
While segmentation makes it possible to visualize the bronchial tree, localization allows us to establish the exact location within the tree. When it comes to assessing and monitoring the bronchi, it is important to assure we can repeatedly quantify the same certain locations within the bronchial tree both longitudinally (between a baseline and follow-up scans) and cross-sectionally (between different patients).
We do this by applying a segmental bronchus labeling algorithm, which identifies 18-22 segmental bronchi locations within the tree, see example below (Figure 3). These are used as anchor points for further quantification, and as generation 0 in many of our delivered biomarkers. Simpler solutions for identifying anchor points in the bronchi (e.g., generation counting) have been proven to be imprecise, especially in the lower lobes, so the localization approach is crucial for reproducibility and consistency of results, no matter which patient or timepoint we are looking at.
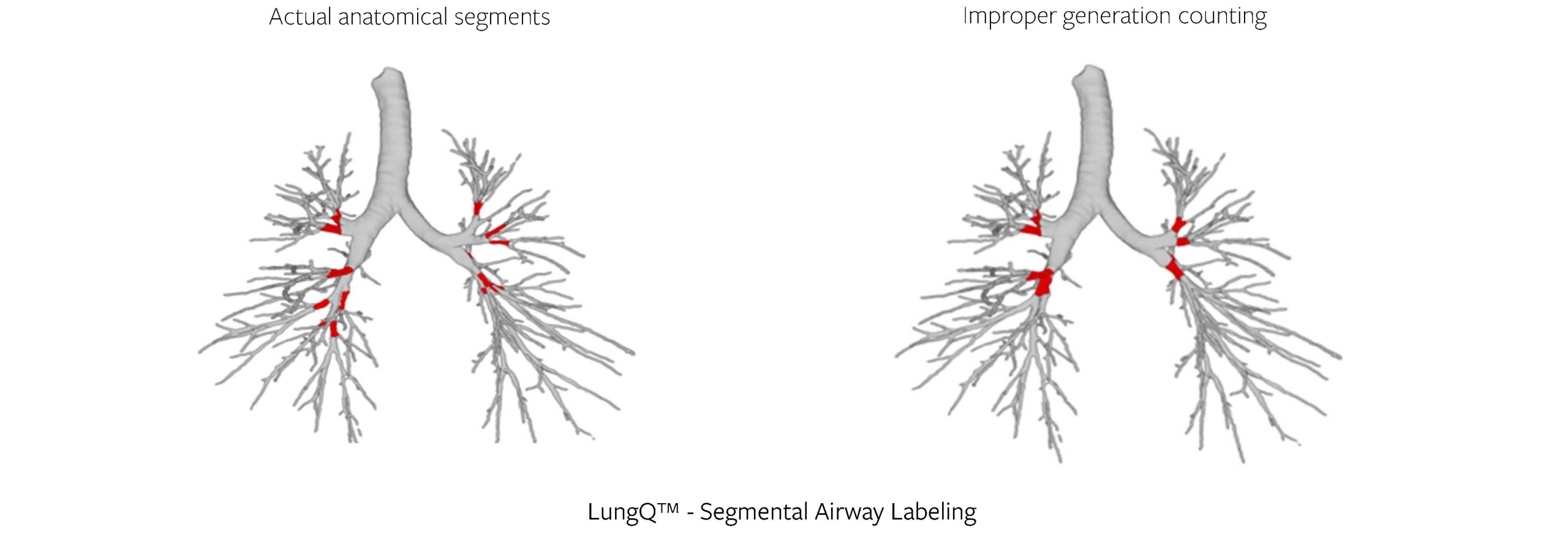
Figure 3
Quantification
The final piece of the puzzle is quantification – and we pride ourselves on this algorithm. While we employ AI in identification and localization, where its superiority is undeniable, we prefer a much more explainable approach when it comes to measurements. We use techniques based on interpolation and intensity integration along the profiles of the identified structures, allowing us to correct for partial volume effects and to measure structures below the resolution of the scan. As a result, we can provide highly accurate measurements of the identified structures, such as bronchial wall thickness, bronchial lumen diameter, and arterial diameter.
Putting it together – powerful biomarkers
Broncho-Arterial (BA) ratios
The Bronchus-Artery (BA) analysis forms the backbone of our LungQ™ Bronchi analysis suite, combining elements of the aforementioned three pillars. LungQ™ BA provides ratios between bronchial wall thickness, inner and outer bronchial diameters and the adjacent artery diameters, for every bronchus generation, starting with the segmental bronchi and going as far into the periphery as the airway segmentation allows (Figure 4).
This is a robust measurement, as it normalizes bronchus dimensions against a reference structure within the scan – the artery. We have seen a great deal of success by applying this measurement in studies in Cystic Fibrosis (CF) and other diseases.
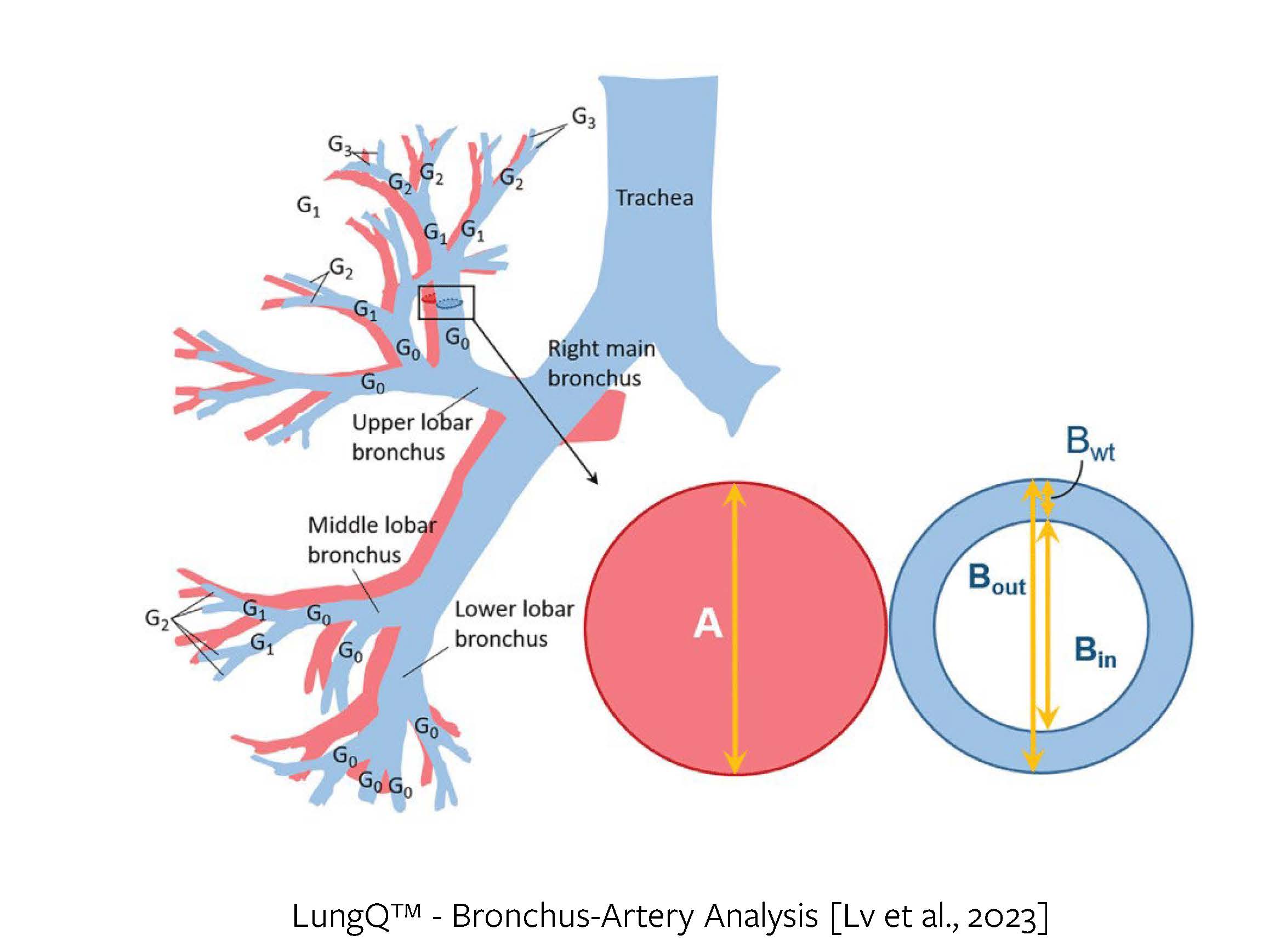
Figure 4
The Bronchus-Artery algorithm is continuously being validated through research studies on different patient and disease cohorts. Amongst the most notable ones is the SHIP-CT study group research on children with Cystic Fibrosis [1], the recently published study in Thorax on bronchus-artery pair detection and progression monitoring in patients with CF [2], as well as the LUM-IVA study on its impacts on children with CF [3].
Independent bronchial measurements
We are also able to measure bronchi independently of the arteries and are exploring biomarkers related to this. The ratio between the wall area and outer airway area (based on outer diameter) was also employed with great success in the SHIP-CT paper [1] as a measure of wall thickness. Standalone bronchi measurements can be used to assess the tapering [4] of the bronchial lumen dimensions, to diagnose bronchiectasis.
What is more – the identified bronchial tree itself provides higher-level metrics that can be indicative of a disease. If a patient has bronchiectasis, more airways will be visible in the scan, thus the identified branch count will be higher. This is an effect that is clearly shown in the World Bronchiectasis Conference paper [4].
Mucus Assessment
Mucus impaction is an important characteristic in many diseases. As shown in the recent paper by Diaz et al. [5], the occurrence of mucus plugs is associated with a higher risk of all-cause mortality in COPD. This points to the fact that assessing mucus can be a crucial advantage in obtaining a well-rounded view of disease processes within the lungs.
LungQ™ conducts mucus impact assessment by measuring bronchial wall thickness with the BA algorithm, as well as automatically detecting occluding mucus plugs within the bronchial tree.
Small Airway Ventilation Assessment (VERA)
Of course, gas exchange in the lungs happens at the alveolar level, and this is a resolution that no CT scan is capable of capturing. While the hitherto mentioned approaches deal only with the visible bronchi, we also have an algorithm that assesses ventilation at a level beyond that – by looking at the inspiratory and expiratory scan pair, with the LungQ™ VERA algorithm we are able to detect areas of hypo-ventilated and/or hypo-perfused lung volume, allowing for assessment of obstructive changes within the small airways which are not visible on CT.
Transforming the treatment approach
Airways play the key role in the lung function as the main pathway of air into our lungs. They are highly heterogeneous between patients and diseases, which makes it important to be able to understand their structure and to objectively assess it when approaching airway diseases. With AI enabling us to monitor diseases longitudinally and cross-sectionally, we can better diagnose and understand disease progression, transforming the way we approach lung diseases and their treatment. It allows us to robustly measure treatment effectiveness over time, establish precise treatment areas and to phenotype patients and diseases.
Through the years of our experience, automated airway analysis has proven its innovative value in a wide array of use cases in pharmaceutical, medical technology and clinical applications. Thirona's AI-based quantification platform consists of a comprehensive portfolio of robust algorithms trained for a variety of different diseases, patient cohorts and input scan characteristics. It has been extensively validated in a multitude of external studies published in over 180 papers.
With our deep scientific roots, we continuously participate in academic research, keeping up our search for new capabilities for the advancement of clinical trials or bronchoscopic and surgical lung interventions, ultimately leading to better patient care, personalized treatments by means of precision medicine.
Contact us if you would like to discuss our airway measurement capabilities in more detail.

