Exploring bronchial anatomical structures, disease progression and mechanism of action
As they play a central role in the functioning of the respiratory system, assessing airways is crucial in lung disease evaluation. Any obstruction, inflammation or narrowing of the airways can impede airflow and cause respiratory problems. Abnormalities such as narrowing (as in asthma), mucus build up (as in chronic bronchitis), or structural changes (as in COPD) can be indicative of certain lung conditions and their corresponding changes over time.
The ability of AI-based algorithms to analyze vast amounts of imaging data with unprecedented precision and accuracy, completely revolutionizes the way we can assess airways to better understand the underlying disease process, conduct personalized interventions and monitor treatment effect.
Fully automatic robust assessment of airway disease
Thirona's Bronchi suite of measurements contains a wide range of robust algorithms, extensively validated in a multitude of external studies, and trained to handle a variety of different patient cohorts and input scan characteristics. Following Thirona's proven 3-step methodology (Identification-Localization-Quantification), we are able to identify subtle structural changes with high precision, accuracy and sensitivity, allowing to monitor disease progression.
The AI-enabled analyses are based on anatomical biomarkers such as bronchial count, wall thickness, lumen diameter, bronchus-artery ratios, mucus plug count and many more. They provide robust quantitative assessment for multiple diseases, as well as the corresponding abnormalities like bronchial dropout, bronchiectasis, mucus impaction, etc.
Identification
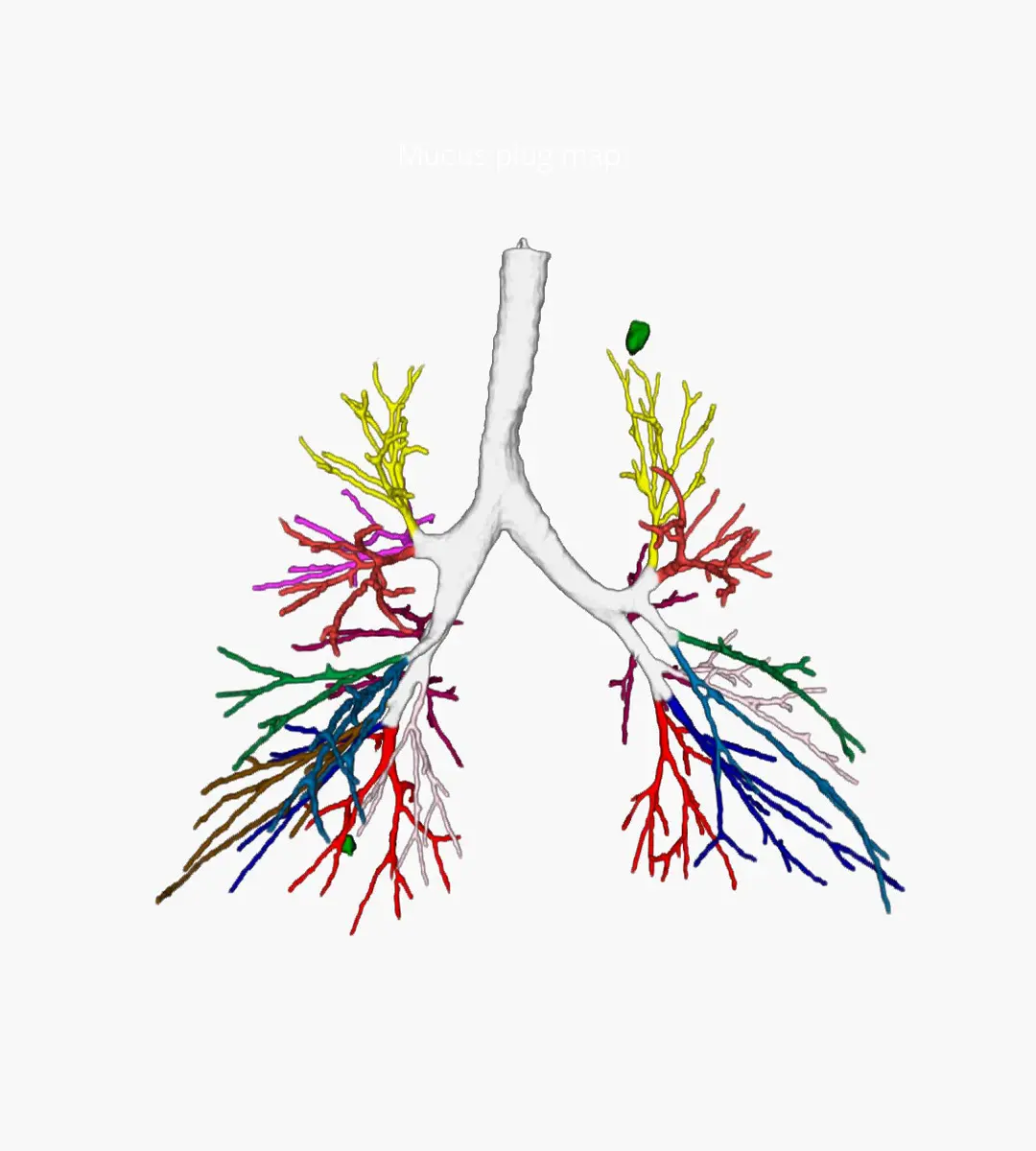
Accurate identification and segmentation of all airways visible on CT to identify the bronchi is a crucial first step in our AI-based analysis. Visualization of the bronchial tree and extracting metrics like branch count, tree length, mucus obstruction as well as locating the adjacent arteries, forms the basis for all following steps.
Localization
While segmentation makes it possible to visualize the bronchial tree, labeling of the exact location within the tree allows us to repeatedly quantify the same locations, both longitudinally and cross-sectionally. Well-conducted localization is pivotal for reproducibility and consistency of results.
Quantification
Our quantification algorithms allow for measuring bronchial structures up to the small bronchi, with high accuracy and precision, even below the scan resolution. Robust quantification of bronchial measurements can be provided on multiple levels, from full lungs to lobar and (sub)segmental analysis, as well as for individual branches and generations.
Enabling imaging-guided lung interventions and pre-/post-treatment assessment
Based on the precisely analyzed and quantified chest CT images, in the context of specific treatment or intervention, Thirona's AI platform can generate a multitude of very detailed visualizations of the bronchi tree and related pathologies.
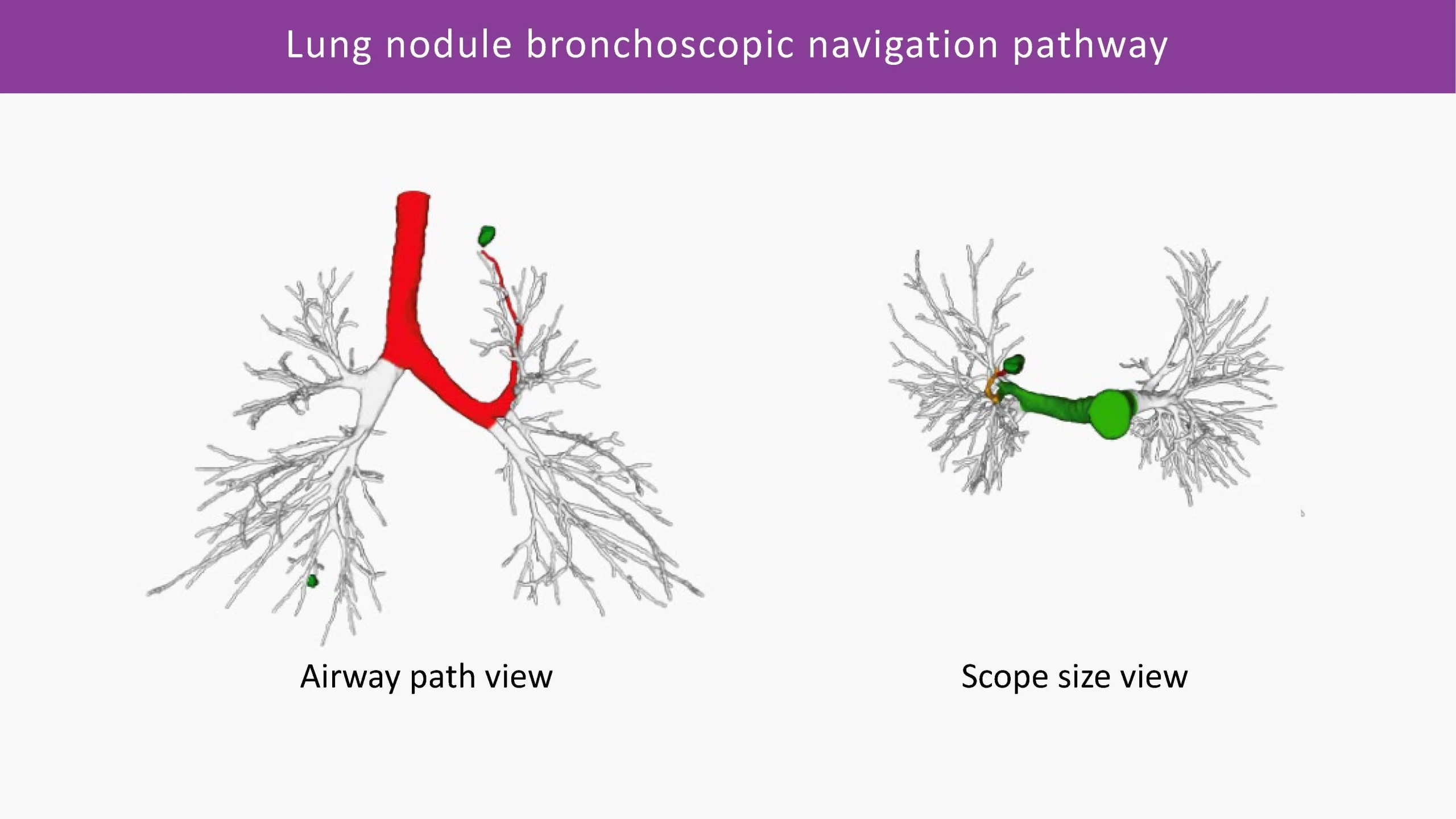
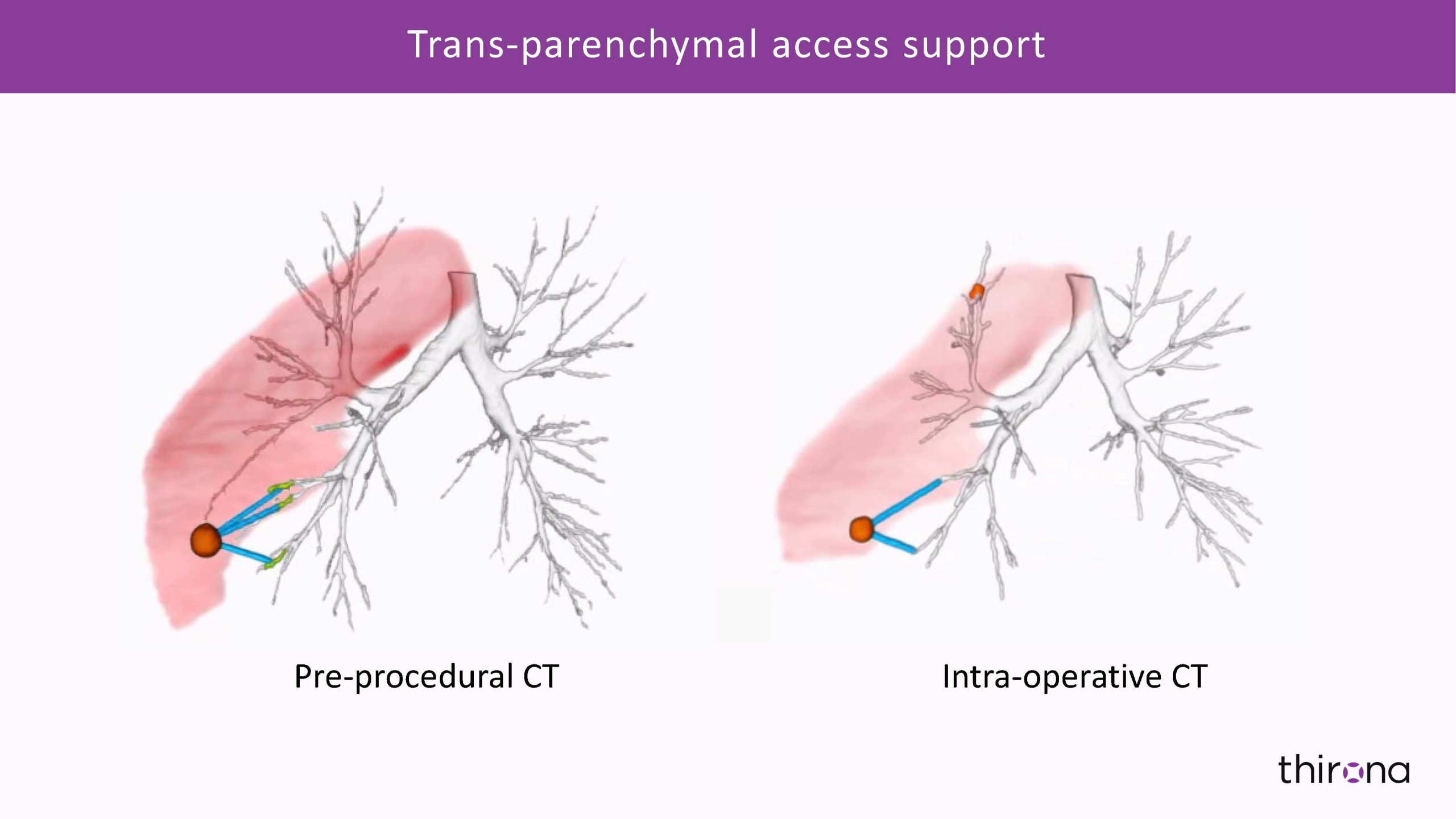
3D visual representation of outcomes, along with the quantitative data report, delivers unprecedented evidence for development of emerging therapies in treatment of airway disease as well as novel insights into lung anatomical structures, enabling next generation surgical and bronchoscopic interventions.
From patient selection, identification of possible treatment pathways and pre-operative navigation planning to intra-operative image analysis, and all the way to objective quantification of treatment efficacy and post-treatment monitoring of disease progression - Thirona's artificial intelligence-based technology fuels innovations transforming clinical paths in pulmonary precision medicine.
Precision medicine applications
The Bronchi suite of analysis offers unique capabilities for determining treatment efficacy for muco-obstructive and other respiratory diseases such as COPD, cystic fibrosis, severe asthma and bronchiectasis. The extended, and robust analysis allows for more accurate patient phenotyping and for sensitive monitoring of longitudinal changes over time.
Accurate segmentation and visualization of bronchial anatomical structures, along with objective quantification of lung abnormalities, can also provide critical insights for bronchoscopic and surgical interventions for the treatment of obstructive diseases (COPD, severe asthma), diseases involving a great deal of mucus obstruction and bronchiectasis (CF, IPF) or lung cancer.
Thirona's major proprietary bronchial measurements
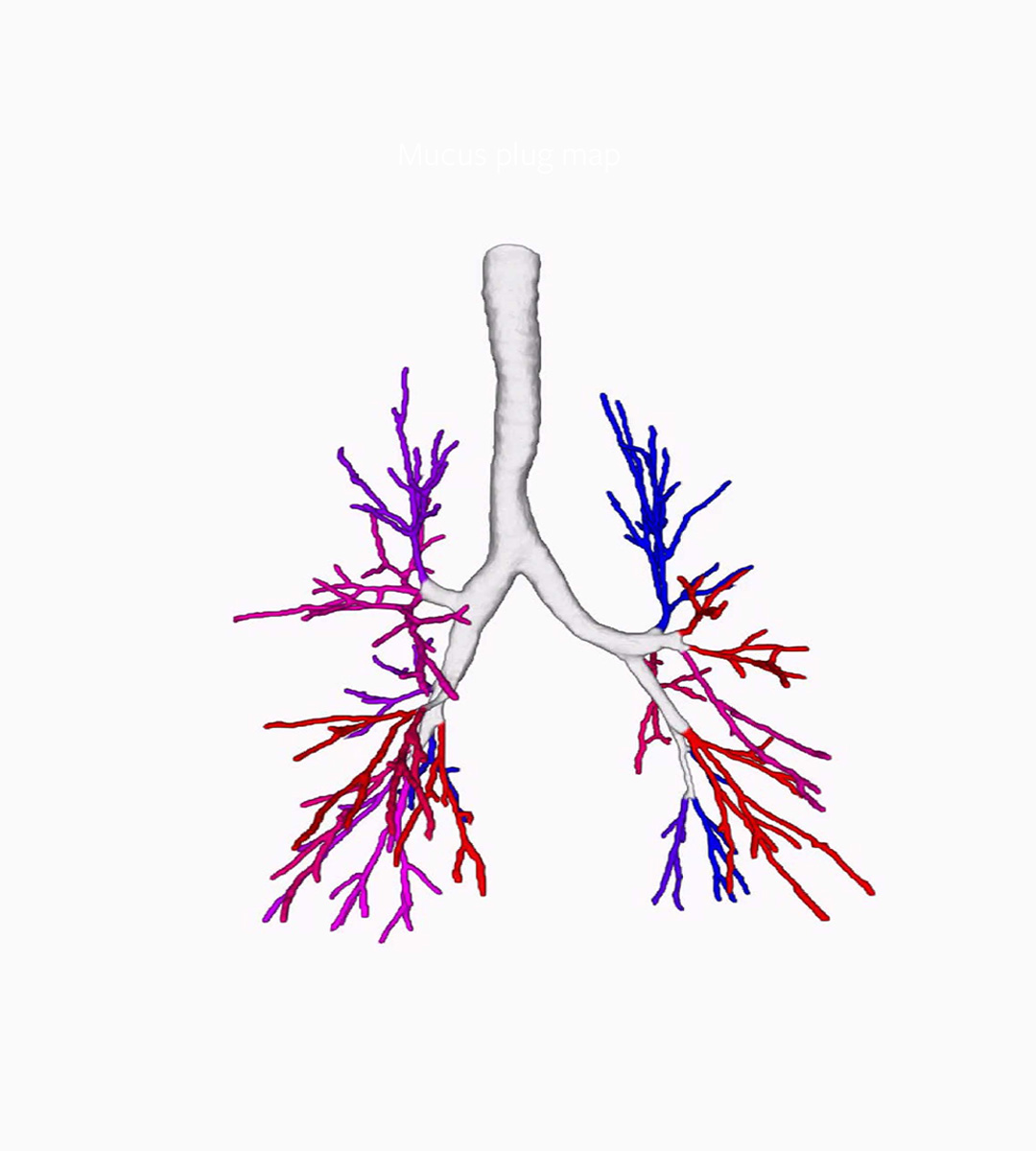
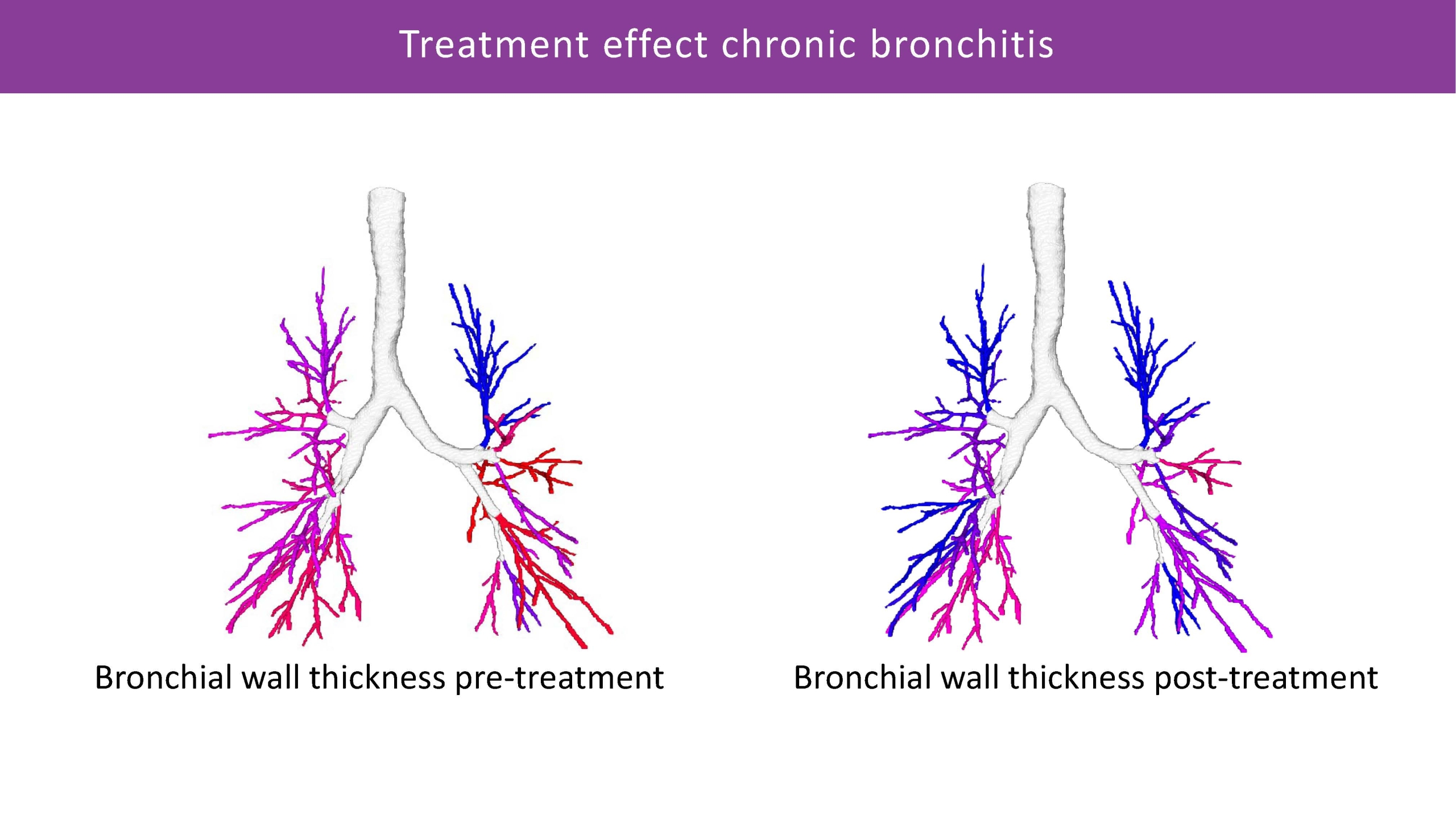
Bronchus-Artery Analysis
Bronchus-Artery (BA) analysis is one of the fundamental measurements of Thirona’s AI-based lung quantification platform. BA conducts an automatic quantification of the entire bronchi tree, while normalizing bronchus dimensions against the adjacent artery as the reference structure within the scan. Providing robust measurements, based on ratios between bronchial wall thickness, inner and outer bronchi diameters, and the artery diameters, LungQ™ BA consistently shows highly reliable performance in cystic fibrosis and a variety of diseases.
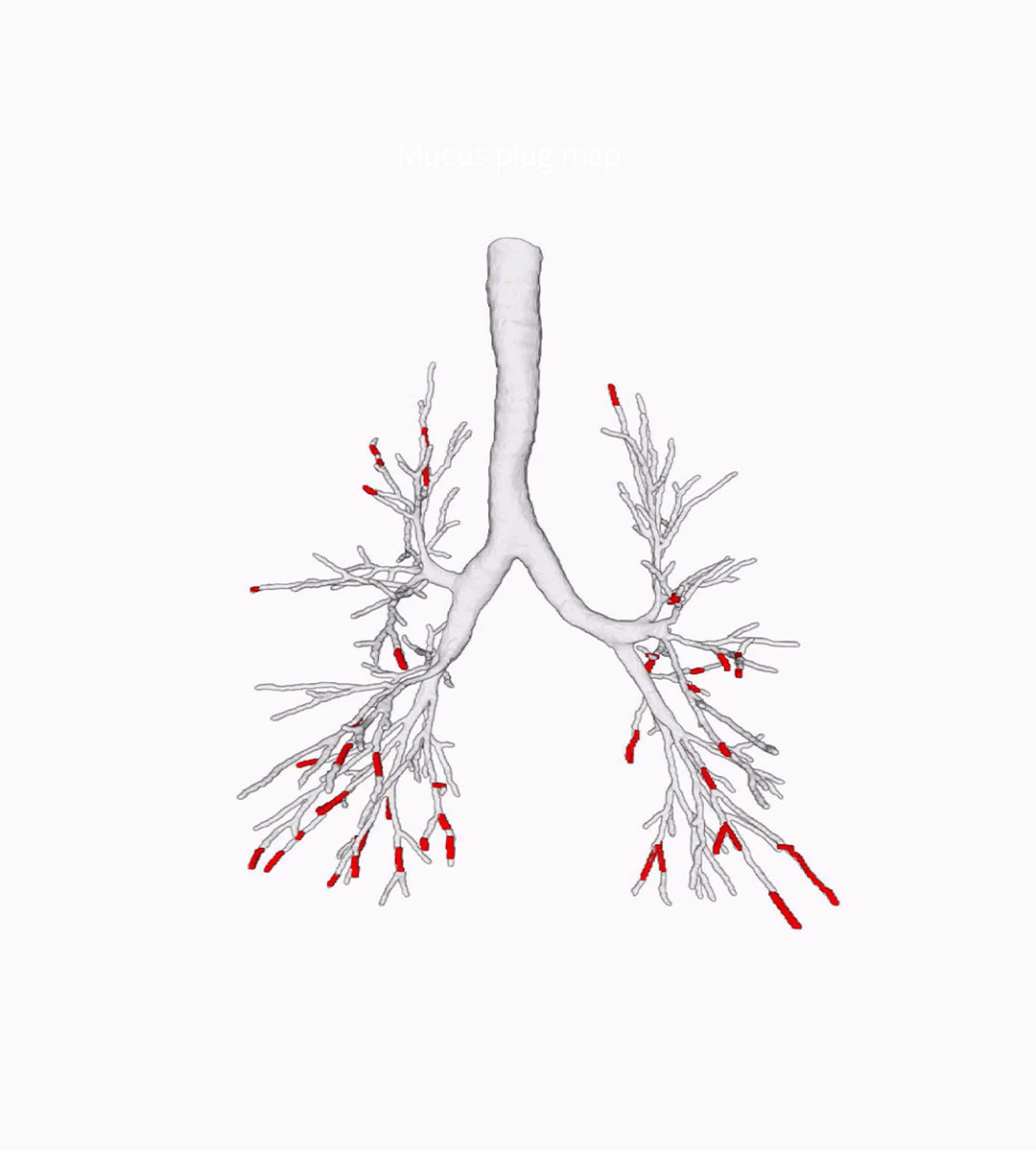
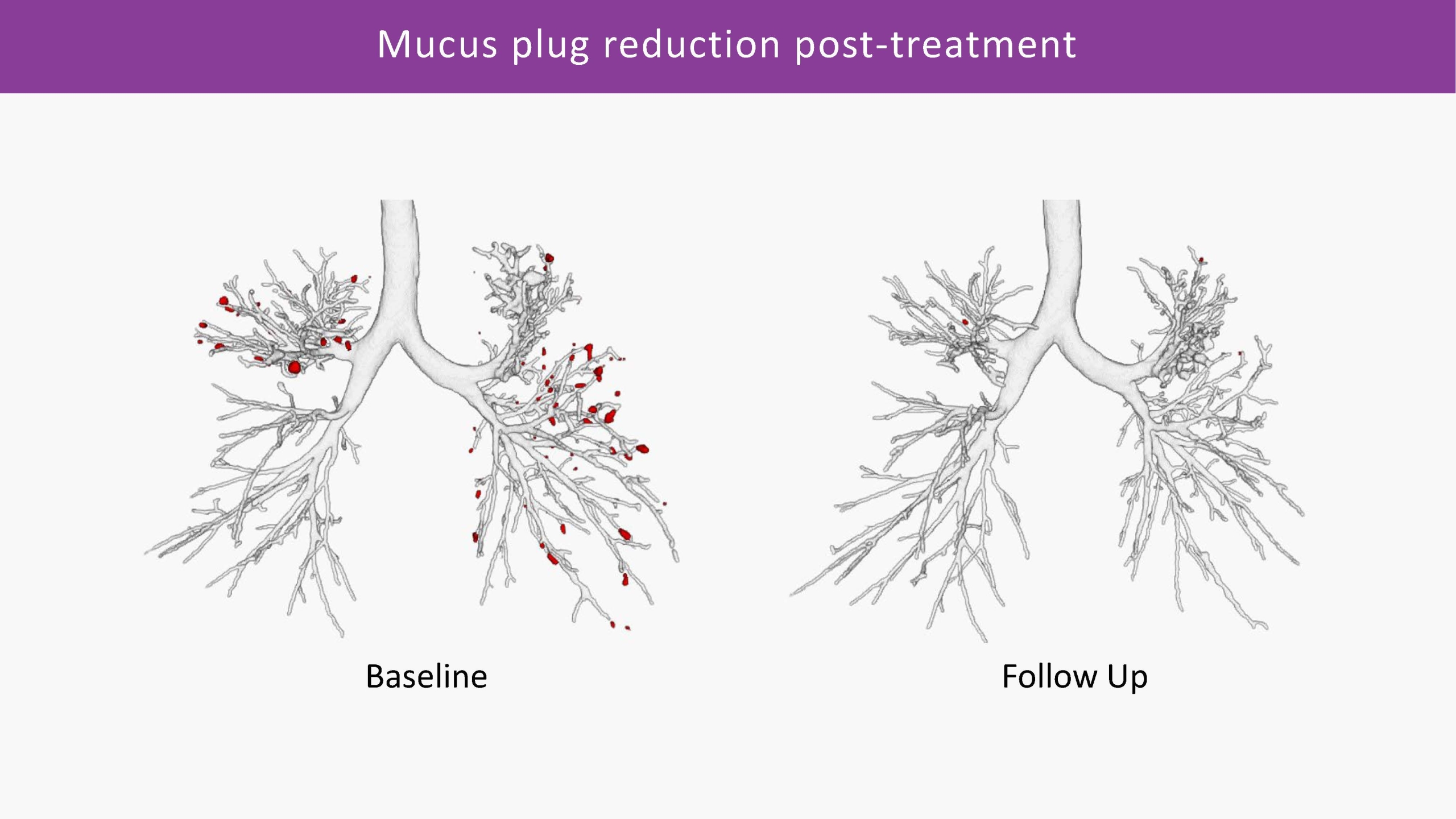
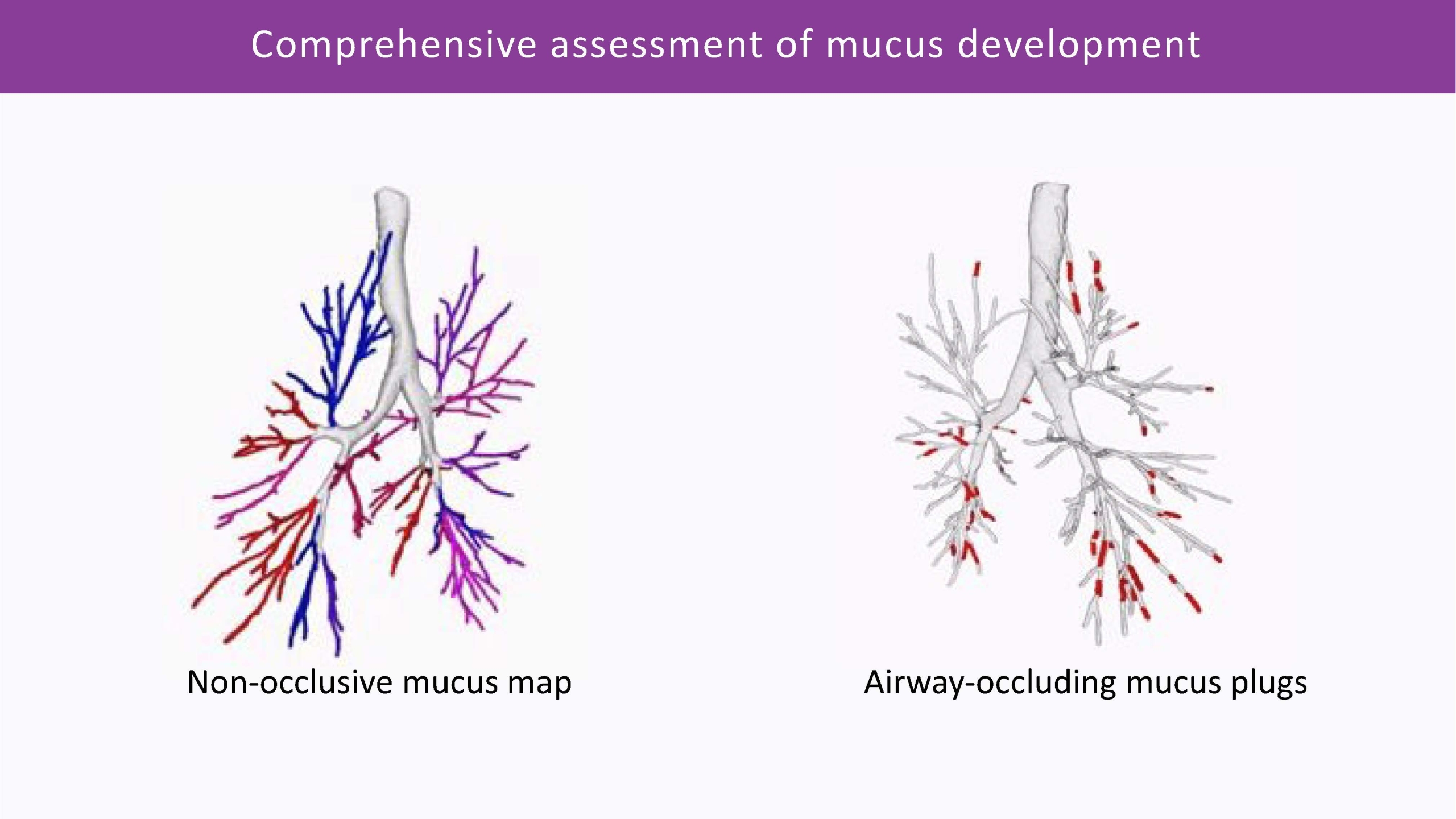
Mucus Plugs Quantification
This algorithm can conduct fully automatic quantification of airway-occluding mucus plugs from CT, throughout the entire bronchi tree. It is the most sensitive way to analyse non-occlusive mucus accumulation: in combination with the Bronchus-Artery analysis, as well as with VERA, which allows for the assessment of small bronchi not visible on CT. We can provide a comprehensive assessment of mucus impaction and distribution, monitoring changes over time with great sensitivity and precision.
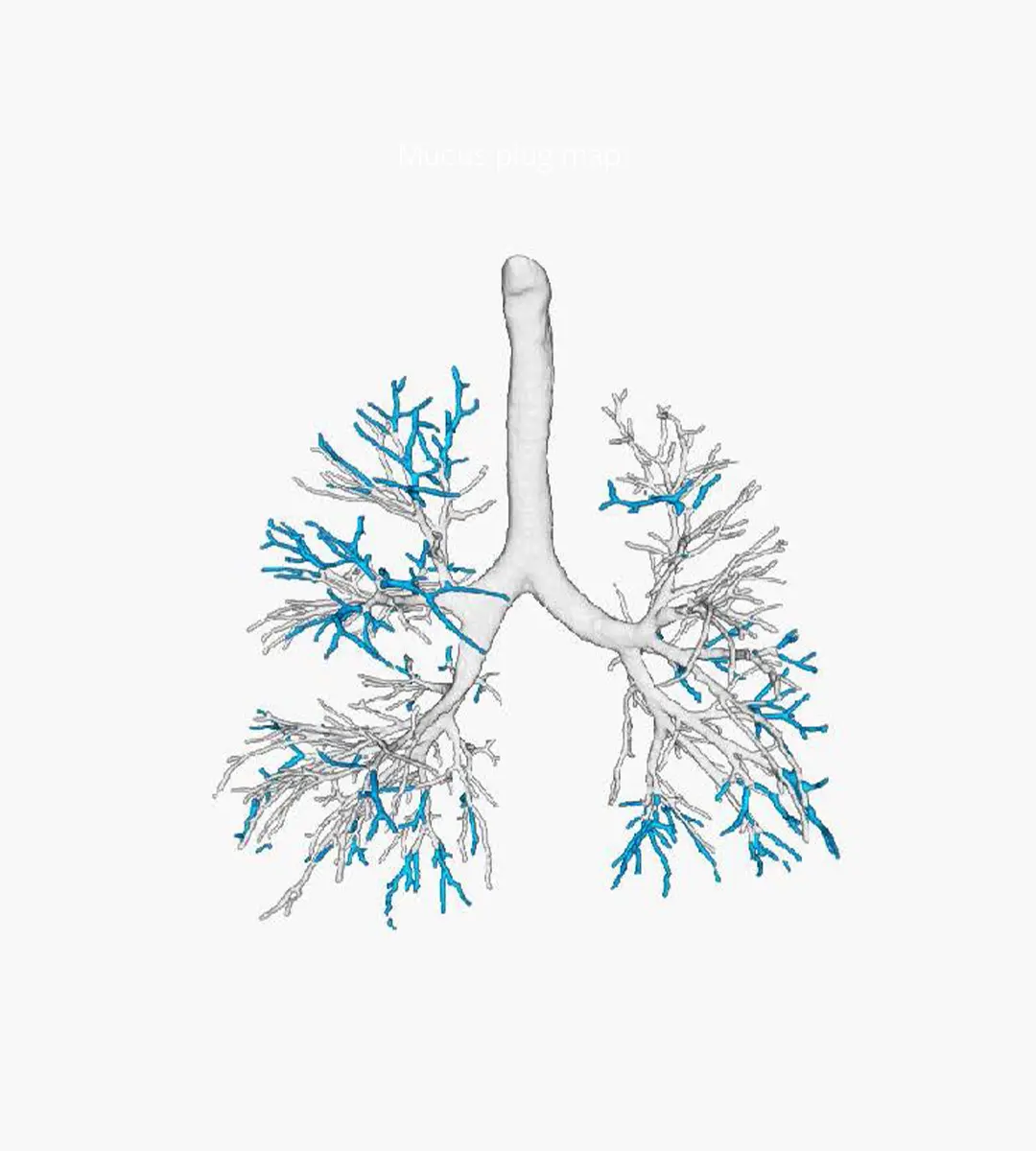
VERA - Ventilation Estimation Small Airway Analysis
VERA algorithm combines inspiratory and expiratory scans to automatically detect areas of hypo-ventilated and/or hypo-perfused lung volume, allowing for the indirect assessment of obstructive changes within the small non-visible airways. The power of this algorithm involves the ability to estimate gas exchange on the alveolar level, a resolution that no CT scan is capable of capturing.
Validation studies and publications
Ongoing external validation studies play a vital role in assessing the ability of our algorithms to perform consistently on diverse patient populations. See below a selection of research studies and publications that speak toward the robustness and clinical applicability of LungQ™ Bronchi analysis.
Cystic Fibrosis
Asthma
COPD
Normal Subjects
Related blogs
How AI-powered quantitative analysis of CT images is redefining bronchiectasis trials and personalized approach to treatment.
Fully automatic image analysis systems enabled by artificial intelligence can now measure the dimensions of almost all airways and blood vessels on a CT scan, with great precision and accuracy.